Cerebri
Home-based biofeedback device for migraine prevention in development
Cerebri has been under development since 2015 with the aim of bringing to market a research-based intervention for preventive migraine treatment. This innovative approach utilizes remote biofeedback techniques to address migraine symptoms and enhance patient care.

Finger sensor & neck sensor
- Evidence
- Randomized controlled trial in progress
- Patients being tested
- More than 300
- Administration
- Non-pharmacological medical device class IIa
- Developed in
- Oslo/Gothenburg
- Patent pending
- EP4161354A1/US2023172540A1
- Devices
- Android/iPhone
Home-based
Traditionally confined to clinical settings, biofeedback therapy will soon be accessible from the comfort of your own living room
Evidence-based
Biofeedback is a scientifically validated learning method, leveraging trial and error to empower individuals to influence their bodily responses over time
Non-pharmacological
Cerebri will offer drug-free preventive treatment for managing migraines, empowering individuals to take control of their condition
The concept
Cerebri is a preventive intervention for migraine treatment, building on years of clinical research
Cerebri is a preventive migraine therapy based on biofeedback. Biofeedback involves measuring bodily parameters, which individuals observe and then learn to influence as part of relaxation therapy. It helps patients monitor and control their stress level in the body.
By using biofeedback, research has already shown that it has a preventive effect on migraines. However, biofeedback is expensive in its traditional format where stationary equipment and a trained therapist is needed. Although biofeedback is already recommended in international guidelines, its availability is limited. Cerebri solves this problem by bringing biofeedback therapy home to patients, making it more affordable and accessible. You can read more about what biofeedback is in our News section.

The innovation
How it works
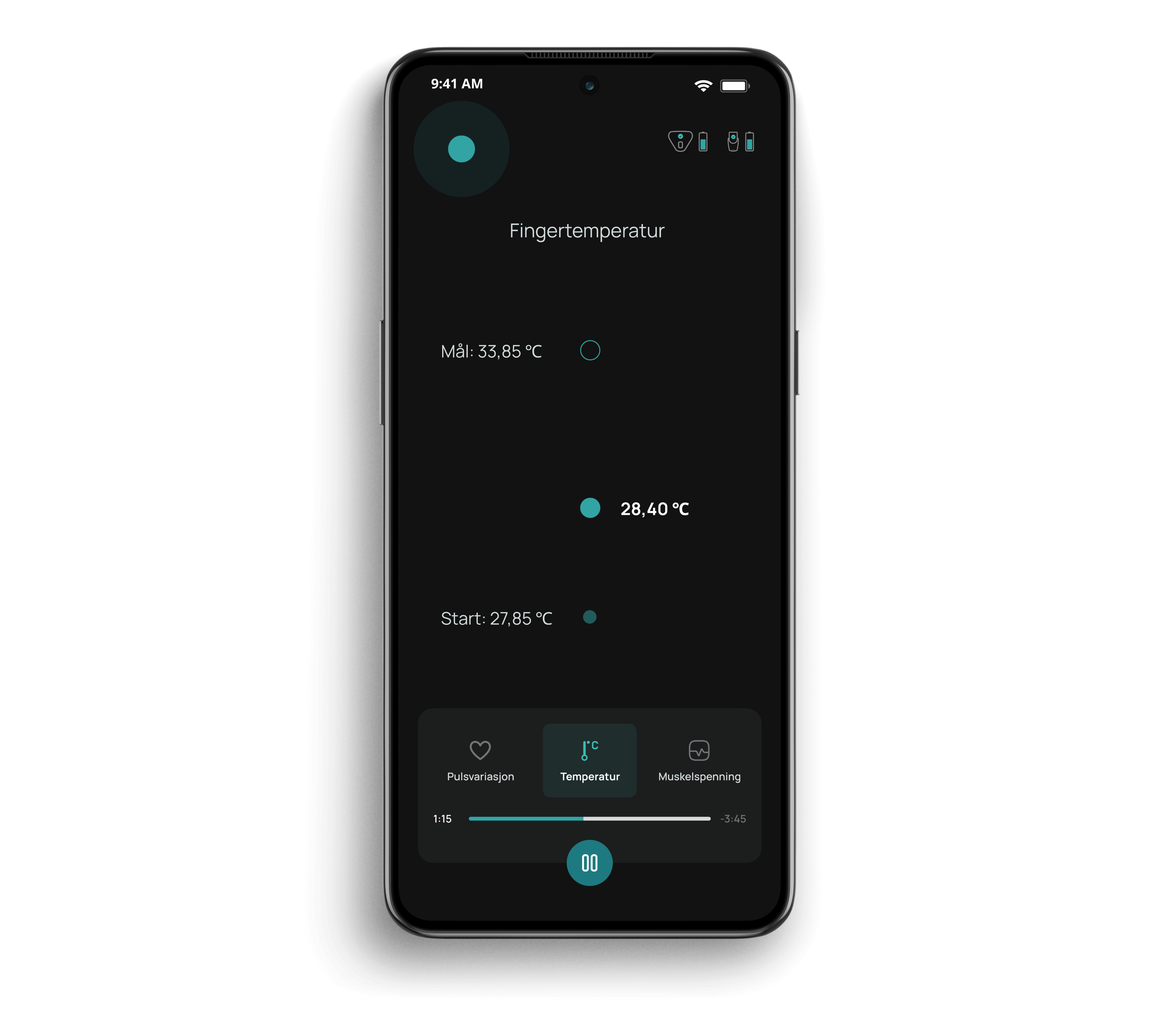
Cerebri utilizes two wireless sensors and a smartphone application to help patients receive biofeedback therapy. The therapy is a self-assisted solution to be used at home for 10 minutes per day for at least 12 weeks. The app is available on both platforms, Android and iPhone, and offers a dark mode option for increased accessibility. The app guides you through the session, and gives instructions on how to breathe and how to reach a biofeedback goal.
Before starting a session with Cerebri, you should find a comfortable and quiet place to sit. You connect the sensors to your mobile phone and follow simple instructions in the app. One sensor is attached to the fingertip to measure temperature and heart rate variability, and the second sensor is attached to the neck to measure tension.
Now keep still, follow a breathing pacer with your breath, watch the measurements and relax. When you complete a biofeedback session after 10 minutes, you remove the sensors and put them away for the next session. That's it!
Short version
- Turn on and attach the sensors to your body
- Start a session to connect sensors to the Cerebri application
- Pay attention to the instructions
- Breathe and relax
- Wait until tomorrow and repeat

The science
Clinical validation
Cerebri is developed as a class IIa medical device (according to the EU Medical Device Regulation - MDR, and ISO 13485), and is built on years of research from the Norwegian University of Science and Technology (NTNU). It all started in 2015, when a systematic review with meta-analysis showed that biofeedback, as a prophylactic treatment for migraine in adolescents, is effective in a traditional clinical setting. Continuing into 2017, wireless sensors measuring peripheral skin temperature and neck sEMG voltage connected via Bluetooth to a smartphone app were compared to traditional neurophysiological “golden-standard” measuring equipment. The project proceeded in 2018 with a usability and development study to assess the usability, feasibility, and safety of the concept. This study also functioned as an experimental proof-of-concept study.
In 2019, a pilot efficacy study on 23 adolescents was performed to investigate the effect size, safety, and tolerability of a therapist-independent biofeedback treatment app among adolescent with migraine. In 2020, a development and usability study was performed to evaluate and improve the feasibility and usability of an mHealth biofeedback treatment app for adults with migraine. A prediction algorithm of migraine was also developed.
In 2022, a pilot clinical trial was performed to test the feasibility, usability, safety, efficacy, and tolerability of Cerebri for adults with episodic migraine (publication in review). In 2023, a randomized controlled trial started to test the effectiveness and safety Cerebri, for migraines in adults. Read more about the trial here.
The device is in the final stages of validation. Claims are to be considered final after the completion of the technical documentation and review by the Notified Body.
List of publications
Scientific publications
Poole A, Winnberg I, Simpson M, Stubberud A, Vetvik K, Bjørk M, Øie L, Holmboe P, Olsen A, Tronvik E, Wergeland T. Feasibility of a 12-Week, Therapist-Independent, Smartphone-Based Biofeedback Treatment for Episodic Migraine in Adults: Single-Center, Open-Label, 1-Armed Trial. JMIR Hum Factors 2025;12:e59622. doi: 10.2196/59622
Poole AC, Stubberud A, Simpson M et al. Biofeedback therapy using Cerebri for the prevention of migraine attacks in adults with episodic migraine (BioCer): a randomized, wait-list controlled trial – the study protocol [version 1; peer review: 1 approved, 1 approved with reservations]. F1000Research 2024, 13:775 (https://doi.org/10.12688/f1000research.149807.1)
Stubberud A, Ingvaldsen SH, Brenner E, et al. Forecasting migraine with machine learning based on mobile phone diary and wearable data. Cephalalgia. 2023;43(5). doi: 10.1177/03331024231169244
Ingvaldsen S, Tronvik E, Brenner E, Winnberg I, Olsen A, Gravdahl G, Stubberud A. A Biofeedback App for Migraine: Development and Usability Study. JMIR Form Res 2021;5(7):e23229. doi: 10.2196/23229
Stubberud A, Linde M, Brenner E, et al. Self-administered biofeedback treatment app for pediatric migraine: A randomized pilot study. Brain Behav. 2021; 11:e01974. doi: 10.1002/brb3.1974
Stubberud, A., Tronvik, E., Olsen, A., Gravdahl, G. and Linde, M. (2020), Biofeedback Treatment App for Pediatric Migraine: Development and Usability Study. Headache: The Journal of Head and Face Pain, 60: 889-901. doi.org/10.1111/head.13772
Stubberud A, Omland P, Tronvik E, Olsen A, Sand T, Linde M. Wireless Surface Electromyography and Skin Temperature Sensors for Biofeedback Treatment of Headache: Validation Study with Stationary Control Equipment. JMIR Biomed Eng 2018;3(1):e1. doi:10.2196/biomedeng.9062
Stubberud, Anker et al. Biofeedback as Prophylaxis for Pediatric Migraine: A Meta-analysis. Pediatrics vol. 138,2 (2016): e20160675. doi:10.1542/peds.2016-0675





